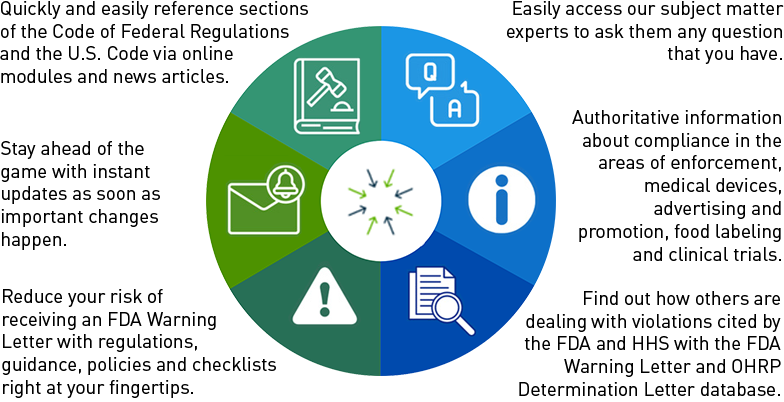
FDA Issues Cancer Trial Guidance on Lab Values, Performance Status, Washout Periods and Concomitant Medications
April 26, 2024 at 02:29 PM EST
The FDA released three more draft guidances on cancer clinical trial eligibility criteria on April 25, bringing the total number of cancer trial eligibility criteria guidances to 13.
The new guidances for sponsors, clinical investigators and institutional review boards deal with laboratory values, performance status and washout periods and concomitant medications.
The labora... Read More